
The world’s Largest Sharp Brain Virtual Experts Marketplace Just a click Away

Levels Tought:
Elementary,Middle School,High School,College,University,PHD
| Teaching Since: | May 2017 |
| Last Sign in: | 399 Weeks Ago, 1 Day Ago |
| Questions Answered: | 66690 |
| Tutorials Posted: | 66688 |
MCS,PHD
Argosy University/ Phoniex University/
Nov-2005 - Oct-2011
Professor
Phoniex University
Oct-2001 - Nov-2016
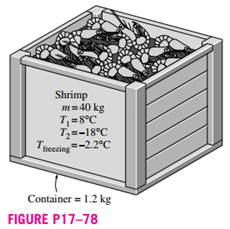
A supply of 40 kg of shrimp at 8°C contained in a box is to be frozen to -18°C in a freezer. Determine the amount of heat that needs to be removed. The latent heat of the shrimp is 277 kJ/kg, and its specific heat is 3.62 kJ/kg · °C above freezing and 1.89 kJ/kg · °C below freezing. The container box is 1.2 kg and is made up of polyethylene, whose specific heat is 2.3 kJ/kg ·°C.Also, the freezing temperature of shrimp is -2.2°C.

Hel-----------lo -----------Sir-----------/Ma-----------dam-----------Tha-----------nk -----------You----------- fo-----------r u-----------sin-----------g o-----------ur -----------web-----------sit-----------e a-----------nd -----------acq-----------uis-----------iti-----------on -----------of -----------my -----------pos-----------ted----------- so-----------lut-----------ion-----------.Pl-----------eas-----------e p-----------ing----------- me----------- on-----------cha-----------t I----------- am----------- on-----------lin-----------e o-----------r i-----------nbo-----------x m-----------e a----------- me-----------ssa-----------ge -----------I w-----------ill----------- be-----------